Flight muscles, Energetics and Behavioral Genetics
Flight muscles and Genetics
The asynchronous indirect flight muscle (A-IFM) of Drosophila is metabolically one of the most active tissues in the animal kingdom. A-IFM delivers the mechanical power to move the wings up- and down and is activated by stretching rather than calcium. There are several mutations affecting function and structure of the adult A-IFM.
Respiratory measurements in tethered flies flying in a flight simulator, allow investigation of how single genes affect muscle performance and efficiency in a behaving organism. Our research shows that muscle efficiency is independent of expression levels of muscle specific proteins. However, mutations of muscle proteins may severely impair muscle mechanical power output of the indirect flight muscle.
Flightin is a multiply phosphorylated, myosin binding protein found specifically in indirect flight muscles (A-IFM) of Drosophila and is important for muscle development and function. A null mutation in the flightin gene (fln0) compromises thick filament assembly and muscle integrity resulting in muscle degeneration and lost of flight ability. Transgenic P[fln+] fln0 rescued flies have less thick filaments per myofbril than wild-type flies but have otherwise normal IFM. Transgenic P[fln+] fln+ ‘tetraploid’ flies have normal number of thick filaments. Flightin expression levels in both transgenic strains are similar to wild-type. Our results suggest that regulation of flightin expression is independent of gene copy number and that the number of thick filaments assembled per myofibril is influenced independently by myosin and flightin expression. Flight parameters at maximum locomotor capacity, measured in a virtual reality flight simulator, are compromised for both transgenic strains. P[fln+] fln0 and P[fln+] fln+ flies generated enough flight force to sustain hovering flight but showed reduced capability to produce forces in excess of hovering flight force. Mean mechanical power output of wild type flies amounts to approximately 70 W/Kg muscle tissue. By contrast, muscle and aerodynamic efficiency are similar among transgenic strains and wild-type.
+++ Check out +++
Lehmann, F.-O., Skandalis, D., and R. Berthé (2013) Calcium signaling indicates bilateral power balancing in the Drosophila flight muscle during turning behavior. J. R. Soc. Interface, 6(10). doi 10.1098/rsif.2012.1050
Flight energetics and Respiration
Insect flight is one of the energetically most demanding forms of locomotion that has evolved in the animal kingdom.
Estimates of maximum locomotor capacity and efficiency of the muscle-mechanical conversion process (muscle efficiency) are vital for our understanding how insects fly. Muscle tissue in flying animals reaches extreme metabolic rates and thus requires an extensive tracheal development. Our research goal is to determine both respiratory capacity and muscle function in behaving Drosophila.The elevated performance values for mechanical power output and muscle efficiency match the extraordinary properties and anatomy of the asynchronous flight muscle in insects. The low density of sarcoplasmatic reticulum typically found in insect flight muscles has freed space for additional contractile filaments and mitochondria. On the one hand, this arrangement seems to enhance muscle mechanical power output. On the other hand, the low SR content makes it difficult to control the power output of these muscles by the nervous system. Flies coped with this trade-off by developing small steering muscles that twitch synchronously with each nerve spike allowing the fly to control wing motion and aerodynamic forces with each single wing stroke.
For questions please contact:
fritz.lehmannuni-rostockde
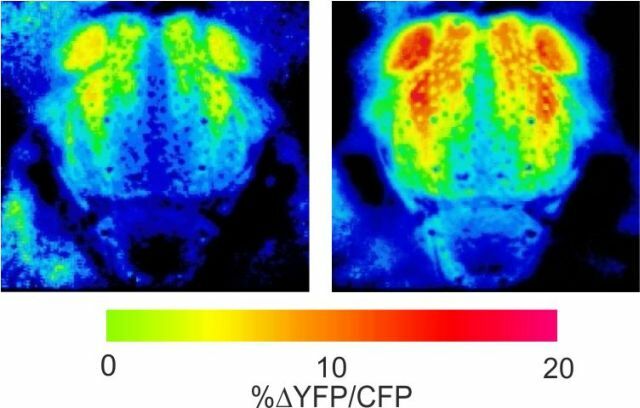
In vivo calcium imaging of flight power muscles
Color-coded calcium activity at rest (left) and flight (right) of a fruit fly.
Calcium probe is Cameleon 2.1 expressed by Mef2. Top view on thorax.